Embryo vitrification doesn't sound much like "freezing embryos,” but the end product certainly is a cryopreserved equine embryo, frozen in liquid nitrogen for the preservation of genetics and transfer into a recipient mare at a later time. For the sake of some readers of this blog, an embryo is the result of fertilizing an oocyte (egg) with sperm and allowing the initial stages of development to occur. Embryo vitrification is the process whereby we freeze equine embryos for storage for indefinite periods of time prior to transfer into a recipient mare. We have the technology to cryopreserve equine sperm and equine embryos, but not equine oocytes. Herein lays the answer to the most common question that comes up in conversation regarding this blog topic. Some readers assume that since we can freeze the male generated sperm, we can likewise freeze the female generated oocyte. Unfortunately, due to some very sensitive cytoskeletal components in the oocyte, the technology does not exist to freeze equine oocytes. As we proceed, you will understand the process of embryo vitrification and we will delve into areas of research that are improving the success rates of vitrified embryos in generating live foals.
Why Freeze Equine Embryos?
 First of all, why would cryopreservation of embryos be such a sought after procedure? Well, by having a cryopreserved embryo, we not only preserve valuable genetic potential from a particular mare and/or stallion combination, but also allow for easy recipient mare management by negating the need for a certain synchrony between the donor and recipient mare at the time of embryo recovery. We can use a specific recipient for a particular donor and even use the donor to carry a foal at a later time when a pregnancy may be more advantageous. Vitrifying embryos also allows for ease of import or export of genetics in the form of embryos, instead of live horses. Lastly, some advanced reproductive techniques such as Intracytoplasmic Sperm Injection (ICSI) from Transvaginal Aspiration of follicles (see our blog article Ovum Pickup in the Mare), can develop more potential embryos than a client may wish to have in any one breeding season. By vitrifying and preserving the extra embryos, pregnancies can be realized over more than one breeding season.
First of all, why would cryopreservation of embryos be such a sought after procedure? Well, by having a cryopreserved embryo, we not only preserve valuable genetic potential from a particular mare and/or stallion combination, but also allow for easy recipient mare management by negating the need for a certain synchrony between the donor and recipient mare at the time of embryo recovery. We can use a specific recipient for a particular donor and even use the donor to carry a foal at a later time when a pregnancy may be more advantageous. Vitrifying embryos also allows for ease of import or export of genetics in the form of embryos, instead of live horses. Lastly, some advanced reproductive techniques such as Intracytoplasmic Sperm Injection (ICSI) from Transvaginal Aspiration of follicles (see our blog article Ovum Pickup in the Mare), can develop more potential embryos than a client may wish to have in any one breeding season. By vitrifying and preserving the extra embryos, pregnancies can be realized over more than one breeding season.
Process of Freezing Equine Embryos
Cryopreservation of any cell requires three processes to occur to have a successful and viable product. Primarily, intracellular water needs to be removed from the cell, so that when any water does freeze, it does not expand and break the fragile phospholipid bilayer of the cellular membrane. Secondly, we need to prevent the ice crystal formation of the fluid external to the cell from causing fracture or crushing of the cell. In essence leaving space between ice crystals for the cells to remain suspended without being crushed. Lastly, we need to make certain that the cryoprotectants do not induce lethal osmotic changes, are toxic to the cells of interest, and that the cooling/thawing process optimizes the health of the cell membrane.
Sounds simple right? Well, an equine embryo isn’t just an ordinary cell. In fact, by the time we recover the embryo at day 6.5 following ovulation, it is not a single cell. The embryo may contain hundreds of cells, surrounded by a glycoprotein capsule, the zona pellucida, and in some cases contains a developing fluid filled blastocoele cavity. Compared to a single sperm cell, the enormity of the equine embryo at day 6.5 is impressive. The differences between an individual sperm cell and a 150 to 250μm morula embryo with ~500 cells make comparison impossible. Likewise, we cannot treat them the same when it comes to cryopreservation. Late in the 1990’s vitrification (photo below of vitrified embryo) proved superior to the slow-freeze style of cryopreservation that we use for sperm and other cell lines.
 Vitrification is glass formation at low temperatures without forming ice crystals. It refers to making a solid, but so quickly, that ice crystals do not form. To accomplish this task, a fast rate of cooling is necessary in the range of -15,000 to -30,000°C per minute. The downside, however, is to tolerate such high rate of cooling, the concentration of cryoprotectants (CP’s) within the embryo must be very high and approaching toxic levels. We try to limit the toxicity of the cryoprotectants by using different types and increasing the concentration gradually, only exposing the embryo to the most toxic levels for the shortest amount of time.
Vitrification is glass formation at low temperatures without forming ice crystals. It refers to making a solid, but so quickly, that ice crystals do not form. To accomplish this task, a fast rate of cooling is necessary in the range of -15,000 to -30,000°C per minute. The downside, however, is to tolerate such high rate of cooling, the concentration of cryoprotectants (CP’s) within the embryo must be very high and approaching toxic levels. We try to limit the toxicity of the cryoprotectants by using different types and increasing the concentration gradually, only exposing the embryo to the most toxic levels for the shortest amount of time.
Commercially, there are manufactured kits containing solutions of cryoprotectants in different concentrations, as well as a sugar based dilution solution for thawing. These kits utilize a stepwise increase in glycerol and ethylene glycol concentration to remove intracellular water and dehydrate the embryo. Unfortunately, with any vitrification protocol, the exposure time to the cryoprotectants is very, very important. Too long in any one media, and the high cryoprotectant concentration kills the embryo. Too little time and equilibration has not occurred and intracellular water remains that will kill the embryo when vitrified.
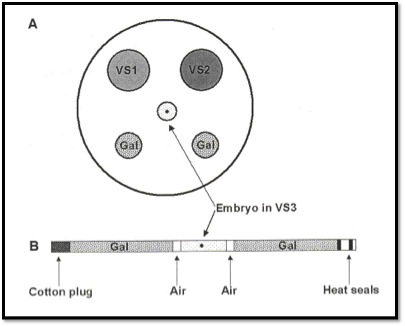
Commercial kits (Bioniche diagram below) also advocate the use of 0.25 ml sterile straws for the loading of the embryo in the vitrification solution of the highest cryoprotectant concentration. To this, we add two columns of sugar based dilution media to help remove the toxic cryoprotectants upon thawing of the straw. The use of a 0.25 ml straw sounds like a small volume, but in the world of vitrification, it is a lot of fluid. Just look at the recent cold weather we have had here on the east coast. What is likely to freeze faster, the 150-gallon stock tank or the thin puddle outside the steps of my front door? The volume of a 0.25 ml straw, plunged into liquid nitrogen at (-196°C) only cools at -2,500°C per minute. That is a far cry from the recommended rate of cooling that exceeds -15,000°C per minute for adequate vitrification.
 These kits do work, and for smaller embryos <300μm in size, pregnancy rates have been reported that approach 75% for vitrified then transferred embryos. This rate of 75% approaches what we expect for fresh embryos, where most transfer success of fresh embryos exceeds 80%.
For embryos larger than 300μm in size, the success rates are significantly lower.
These kits do work, and for smaller embryos <300μm in size, pregnancy rates have been reported that approach 75% for vitrified then transferred embryos. This rate of 75% approaches what we expect for fresh embryos, where most transfer success of fresh embryos exceeds 80%.
For embryos larger than 300μm in size, the success rates are significantly lower.
Freezing Larger Embryos
Vitrifying larger embryos is not a viable technique due to the low transfer success. Yet managing mares to recover embryos when they are <300μm requires accurate timing of ovulation so that you flush the mare for embryo recovery 6.5 days later. We do these early flushes in an attempt to get small embryos. We know the embryo leaves the oviduct and enters the uterus around day 5.5. However, we are bound to miss embryos that have a delay in entering the uterus. Increasing the difficulty is the rapid growth of an equine embryo at this stage of development. Even being 8-12 hours late with a flush can be the difference of recovering an appropriate sized embryo versus one too large to vitrify.
Techniques have been developed in the laboratory using microscopes with micromanipulators, embryo holding capabilities, and specialized mechanisms (Piezo Drill) to make small holes in the embryo to help bypass the capsule that prevents the influx of cryoprotectants and also helps by removing blastocoele fluid that further compromises the success of large embryo vitrification. These techniques hold promise for not only improving the success of vitrifying larger embryos, but also screening the embryo for genetic diseases and embryo sex determination. However, the equipment required only exists at a few specialized centers around the country and world. The experience of the operator to perform these procedures directly influences the successful outcomes.
Lastly, there are two areas where we have made major increases in the success of the embryo vitrification. The first is one of good science while the second is a bit fortuitous. As I mentioned earlier, 0.25ml straws are a large volume to vitrify. By decreasing the volume as well as increasing the surface area, we can greatly increase the cooling rate of the procedure. Using a thin disc (CryoLoop) or thin pulled straws enables us to decrease the amount of vitrification media surrounding the embryo. In the case of the CryoLoop, the embryo is suspended in a thin film of cryoprotectant and vitrified. When using the pulled straws, the volume of fluid is very low, and allows for a more appropriate cooling rate. Using either the CryoLoop or the pulled straws, we thaw the embryo directly in a sugar based warm dilution solution to decrease the concentration of ethylene glycol and glycerol. These steps have increased the successful transfer rate of the thawed-transferred embryos.
In the laboratory (in vitro), ICSI produced equine embryos do not grow a capsule. The glycoprotein capsule is a major impediment to CP entry into the equine embryo. So a combination of lacking a capsule and careful (daily) evaluation as to size, morphology, and developmental stage of the embryo, ICSI produced embryos are vitrified at the precise moment their size and developmental stage are optimal. Couple this perfect timing with a lack of capsule (and very good veterinarians in pristine, climate controlled clean rooms), ICSI produced embryos have the best chances of being vitrified at the correct time for optimal post thaw results.
The take home message is that even though vitrification of embryos sounds like a wonderful plan to generate embryos to be transferred in the future, laboratory success and real world success often do not correlate 100%. I tell clients to expect a 75-80% success rate for the transfer of fresh embryos recovered from the donor that day. If we were to vitrify the same embryos for later transfer I would tell them to expect about at 50-60% transfer success. Vitrifying an embryo will decrease the chances of survival of that embryo when transferred. Other variables such as donor age, fertility, and embryo size may also affect the transfer success of the embryo following vitrification. It is up to each owner to discuss with their veterinarian regarding the pros and cons of each patient and determine what is in the best interests of the owner, the mare, and the future offspring.
If you like this article you may be interested in:
Choosing the Right Recipient Herd


Log in to join the conversation.